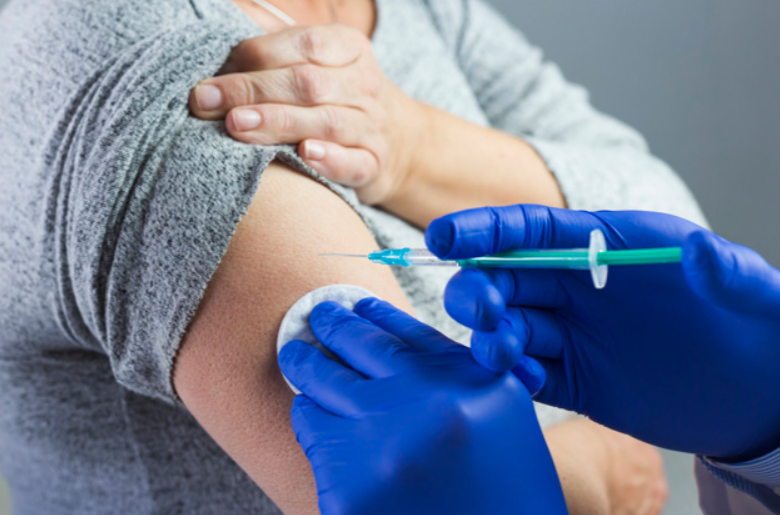
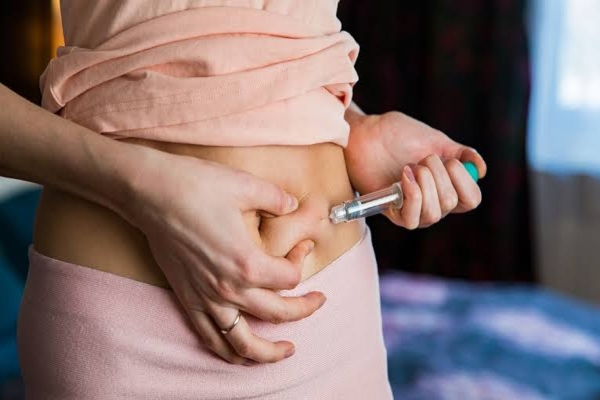
Vaccines are one of the foremost effective options in preventing various diseases. They help the body’s system to acknowledge and fight against viruses or bacteria by training our body system to form antibodies, whilst it does when it’s exposed to a disease.
WHO estimates that vaccines save between 2 and three million lives per annum. Nearly 20 million globally still miss out on the vaccine putting them in danger of great diseases, disability, death, and unhealthy.
Method Of Consumption
- Injections
- Orally (given into the mouth)
- Nasal (sprayed into the nose)
Why does it take time to develop the vaccine?
Vaccine development is usually complex and takes time because it involves a multistep process that has rigorous clinical testing and regulatory procedures.
Step 1: Identify the pathogen
The biological pathogen is identified, isolated, and analyzed by the experts.
Step 2: Pre-clinical stage
The vaccines are developed by growing pathogens that are either inactive or reduced in potency and they are tested upon the animals to look for any potential side-effects.
Step 3: Human Clinical Trials
Human Clinical trials usually involve 3 steps
• Phase 1:Open Label Testing of vaccine on animals
Potential vaccines are given in small doses to healthy human volunteers. this is often designed to form sure the vaccine is safe while determining the right dosage. Phase 1 studies rarely have quite 100 people and lots of begin with fewer than twenty-four volunteers. Phase 1 trials usually take a minimum of a year.
• Phase 2: Blind Studies of vaccine’s effect
During phase 2 trials, a long time dosage is given to subjects to make a decision on how reproducible and consistent the immune reaction is and to spot possible side-effects. Usually, scientists conduct several phases of two studies involving a couple of hundred patients per trial. This process can take upto 2-3 years.
• Phase 3: Random testing of the vaccine
It involves testing the vaccine on thousands of individuals to screen rare side effects.
• Phase 4: Keeping a track of the vaccine
After a vaccine is in the market, prescribers monitor its use for potential adverse reactions that are reported to the manufacturers to make sure its continued safety.
Step 4: Regulatory Hurdles
Before a vaccine is often distributed to the overall population, it undergoes scrutiny from government regulators.
Step 5: Manufacturing and Distribution
Once a vaccine is approved, it must be produced in huge quantities to supply immunity to an outsized number of individuals across the country. Hence, the manufacturing of the vaccines requires specialized equipments and skilled laborers. Ultimately, creating a completely new vaccine may be a challenging and lengthy process. Indeed, there’s no guarantee of success.